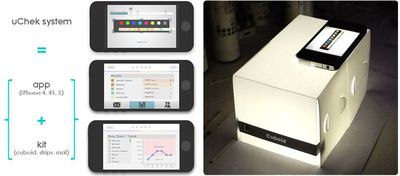
iPhone Urinalysis App Draws U.S. Government Scrutiny
The U.S. Food and Drug Administration has sent a letter to BioSense Technologies over its iPhone uChek urinalysis system, asking why its medical app hasn't been cleared by the agency. The app is one of the first that turns the iPhone into a medical device, designed to read urinalysis test strips that are normally examined by users and compared to a color-coded chart.
With the uChek system, patients can take a picture of the strip with the iPhone's camera and then receive an automated readout of parameters like glucose, urobilinogen, pH, ketone and more. The app also stores results which then can be analyzed over time.
Though medical device makers have adopted the iPhone for some measurements like blood glucose monitoring for diabetics, large scale use of smartphones and tablets as a replacement for existing medical devices has yet to take off -- likely due in large part to government regulation of medical devices.
From Bloomberg:
Biosense Technologies Private Ltd.’s uChek system isn’t cleared by the Food and Drug Administration and the agency said it wants to know why not, in a first-of-its-kind letter to a maker of a mobile-device application. The app relies on users, such as diabetics checking their glucose, to dip test strips in urine and use the smartphone’s camera to allow the system to processes and generate automated results.
UChek works with test strips made by Siemens AG (SIE) and Bayer AG (BAYN), which are only approved for visual reading and require new clearance for automated analysis, the FDA said in the letter. The agency has said it wants stricter rules for apps that directly diagnose or treat conditions, proposing in 2011 to apply similar quality standards as for heart stents, ultrasound machines and other medical devices.
The uChek kit can be purchased in the US and India for $40, while the uCheck iPhone app is a free download [Direct Link] from the App Store -- though the app can also manually read urine strips from other companies.
Popular Stories
Apple plans to announce the iPhone 17e on Thursday, February 19, according to Macwelt, the German equivalent of Macworld.
The report, citing industry sources, is available in English on Macworld.
Apple announced the iPhone 16e on Wednesday, February 19 last year, so the iPhone 17e would be unveiled exactly one year later if this rumor is accurate. It is quite uncommon for Apple to unveil...
Apple turns 50 this year, and its CEO Tim Cook has promised to celebrate the milestone. The big day falls on April 1, 2026.
"I've been unusually reflective lately about Apple because we have been working on what do we do to mark this moment," Cook told employees today, according to Bloomberg's Mark Gurman. "When you really stop and pause and think about the last 50 years, it makes your heart ...
Apple today shared an ad that shows how the upgraded Center Stage front camera on the latest iPhones improves the process of taking a group selfie.
"Watch how the new front facing camera on iPhone 17 Pro takes group selfies that automatically expand and rotate as more people come into frame," says Apple. While the ad is focused on the iPhone 17 Pro and iPhone 17 Pro Max, the regular iPhone...
In the iOS 26.4 update that's coming this spring, Apple will introduce a new version of Siri that's going to overhaul how we interact with the personal assistant and what it's able to do.
The iOS 26.4 version of Siri won't work like ChatGPT or Claude, but it will rely on large language models (LLMs) and has been updated from the ground up.
Upgraded Architecture
The next-generation...
While the iOS 26.3 Release Candidate is now available ahead of a public release, the first iOS 26.4 beta is likely still at least a week away. Following beta testing, iOS 26.4 will likely be released to the general public in March or April.
Below, we have recapped known or rumored iOS 26.3 and iOS 26.4 features so far.
iOS 26.3
iPhone to Android Transfer Tool
iOS 26.3 makes it easier...




















